Mizoribine Effective in Lupus Nephritis Save
A randomized clinical trial has shown that oral mizoribine, a common immunosuppressant in Japan, was noninferior to intravenous cyclophosphamide (CTX) as induction therapy in patients with active lupus nephritis.
Mizoribine (MZB) is an imidazole nucleotide that is a selective inhibitor of inosine-5-monophosphate dehydrogenase, an enzyme crucial to purine synthesis, and as such can suppress the proliferation of T and B lymphocytes. MZB is been approved in Japan for use in lupus nephritis, rheumatoid arthritis, and primary nephritic syndrome, and is often used in combination with corticosteroids. As a purine synthesis inhibitor, this immunosuppressive has less toxicity than azathioprine, and like mycophenolate, has been used in renal transplantation. MZB is not approved for use by the FDA or EMA.
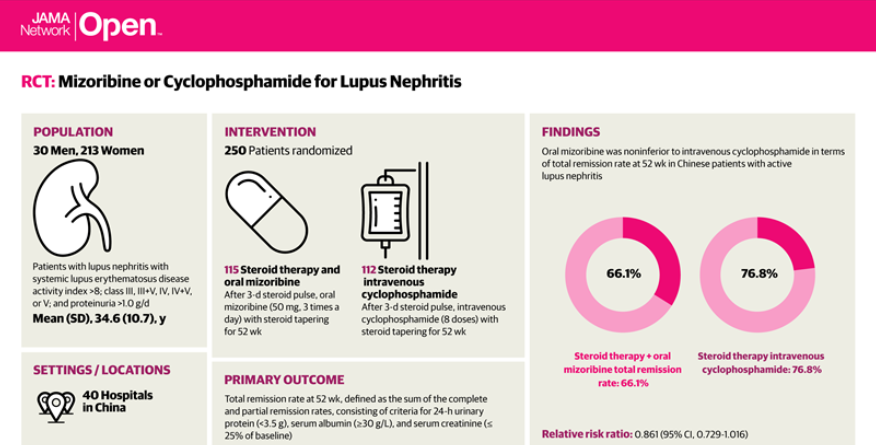
This trial set out to assess the efficacy and safety of oral mizoribine vs intravenous cyclophosphamide as induction therapy for Chinese patients with lupus nephritis. This prospective, multicenter, open-label, phase 3 randomized clinical trial recruited adult lupus patients with class III, III+V, IV, IV+V, or V lupus nephritis with 24-hour urinary protein levels greater than 1.0 gram/day and and a systemic lupus erythematosus disease activity index (SLEDAI) of 8 or higher. The primary endpoint was total remission rate (complete remission rate plus partial remission rate) after 52 weeks.
Oral mizoribine (50 mg, 3 times a day) or cyclophosphamide (6 intravenous doses at 0.5-1.0 g/m2 body surface area, with a maximum dose of 1.0 g/d) for 52 weeks plus oral glucocorticoid.
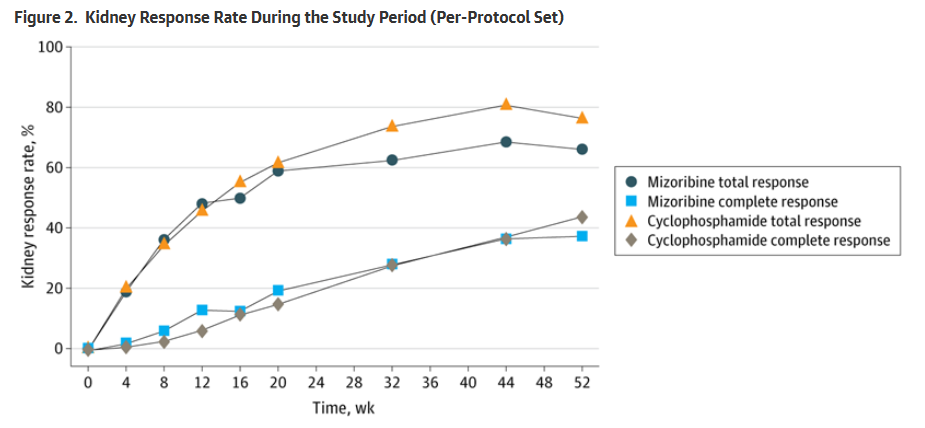
A total of 243 patients were randomized and treated (mean age, 34.6 years, 88% women. The total remission rates at 52 weeks were:
- MZB = 66.1%
- CTX = 76.8%
- Relative risk ratio (MZB vs CTX) was 0.861 (95% CI, 0.729-1.016).
The lower limit of the noninferiority margin was 0.726, Thus, mizoribine was noninferior to cyclophosphamide. Changes in other immune parameters and kidney function were generally similar between the groups.
Adverse events (AE) were generally similar between groups (80.5% MZB vs 78.7% CTX). There were more serious AEs with MZB (27.6% vs 18%), including more serious infections (12.2% vs 10.7%). Leukopenia was more commone with CTX (3.3% vs 1.6%) during the study.
When used with glucocorticoids for induction therapy of active lupus nephritis, oral MZB was an effective alternative to intravenous CTX.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.