Rheumatic Events With Checkpoint Inhibitors: Tumor Type Matters Save
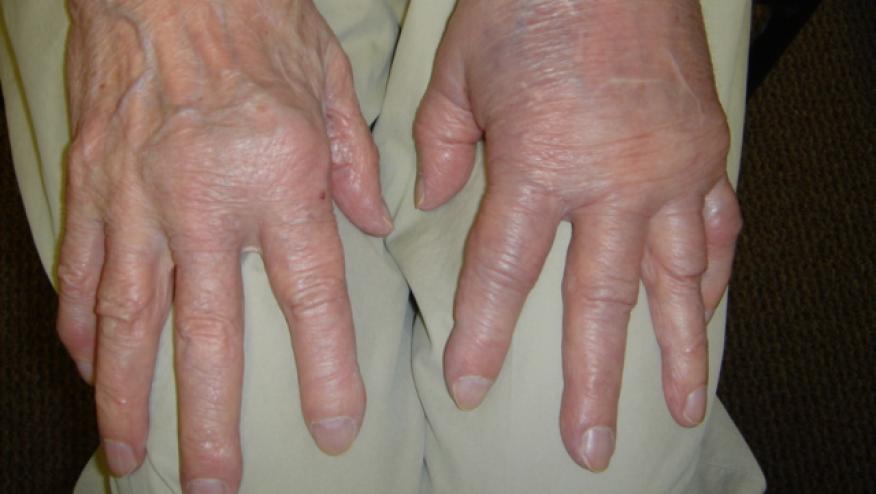
Among the factors that were associated with an increased risk for the development of rheumatic immune-related adverse events following cancer treatment with immune checkpoint inhibitors (ICIs) was the type of malignancy involved, a large case-control study found.
Compared with reference lung cancer (the most common type of malignancy in this cohort), the development of a rheumatic immune-related adverse event was associated with a diagnosis of melanoma, with an odds ratio (OR) of 4.06 (95% CI 2.54-6.51), or genitourinary cancer (OR 2.22, 95% CI 1.39-3.54), reported Jeffrey A. Sparks, MD, of Brigham and Women's Hospital and Harvard Medical School in Boston, and colleagues.
For de novo inflammatory arthritis specifically, similar results were seen, with ORs of 2.91 (95% CI 1.58-5.35) for melanoma and 2.36 (95% CI 1.32-4.19) for genitourinary cancer, the researchers noted online in Arthritis & Rheumatology.
Immune-related adverse events, which can affect virtually any organ system, have increasingly been reported among cancer patients receiving ICI treatment. For patients who develop refractory symptoms, subspecialty evaluation and immune-modulating therapy may be needed.
The most common rheumatic immune-related adverse event is new-onset inflammatory arthritis, which has been reported in 2% to 7% of all patients receiving ICIs. However, it has not been determined whether there are baseline predictors of these events or how often referral to rheumatology occurs or immune-modulating treatment is required.
Accordingly, the researchers conducted a retrospective study of patients with cancer seen at Mass General Brigham or the Dana-Farber Cancer Institute in Boston from 2011 to 2020 who had received ICI therapy.
A total of 8,028 patients had been treated with ICIs, with the most common cancer types being lung cancer, in 31.2%, melanoma in 21.3%, and genitourinary cancer in 15%. The most frequently used ICIs were pembrolizumab (Keytruda) and nivolumab (Opdivo).
A chart review revealed that among these patients, 226 developed rheumatic adverse events, such as arthritis, arthralgia, psoriatic arthritis, or dermatomyositis. These were matched with 2,312 controls who had no pre-existing rheumatic disease and did not develop immune-related adverse events.
Among the ICI-treated patients, 4.9% had a pre-existing rheumatic disease, and 24.1% had other types of autoimmune diseases. Systemic corticosteroid use was reported among 37.2% of ICI-treated patients during the previous year, usually with chemotherapy.
Referral to a rheumatologist occurred in 404 patients, with almost half being diagnosed with an immune-related adverse event, most often (29.2%) de novo inflammatory arthritis. One-quarter of patients were determined to have a pre-existing rheumatic disease, and their symptoms were considered a disease flare. In one-third of patients, two or more types of immune-related adverse events were reported.
A total of 475 patients received an immune-modulating drug after initiating ICI treatment. The most commonly used medications for patients who developed arthritis were hydroxychloroquine, infliximab (Remicade), methotrexate, and sulfasalazine.
The case-control analysis looking for predictors of rheumatic immune-related adverse events adjusted for age, sex, race, type of ICI, pre-existing autoimmune disease, comorbidities, and previous use of glucocorticoids or immune modulators.
Along with the type of cancer, baseline factors associated with the development of these adverse events included:
- Combination ICI therapy versus monotherapy: OR 2.35 (95% CI 1.48-3.74)
- Pre-existing non-rheumatic autoimmune disease: OR 2.04 (95% CI 1.45-2.85)
- Use of a corticosteroid in the previous year: OR 2.13 (95% CI 1.51-2.98)
Baseline variables that were associated with referral to a rheumatologist included a diagnosis of melanoma or genitourinary cancer, pre-existing rheumatic disease, and previous use of an immunomodulator. Baseline predictors of treatment following ICI initiation with an immunomodulator included melanoma, combination ICI therapy, pre-existing rheumatic or non-rheumatic autoimmune disease, and previous use of an immunomodulator.
In discussing their findings, the researchers noted that melanoma and genitourinary cancers are considered "inflammatory tumors" that are likely to respond to ICIs. "Inflammatory tumors have high numbers of somatic mutations within the tumor genome and express immunogenic, aberrant proteins referred to as tumor neoantigens. Thus, an intriguing hypothesis is that increased immune recognition of neoantigens within the tumor may culminate in a higher burden of inflammation at distant tissues, clinically recognized as immune-related adverse events," they explained.
A limitation of the study was the lack of patient-level data on some variables such as subtypes of cancer, which could result in residual confounding.
Comment









If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.