Risankizumab Outduels Secukinumab in Psoriasis Save
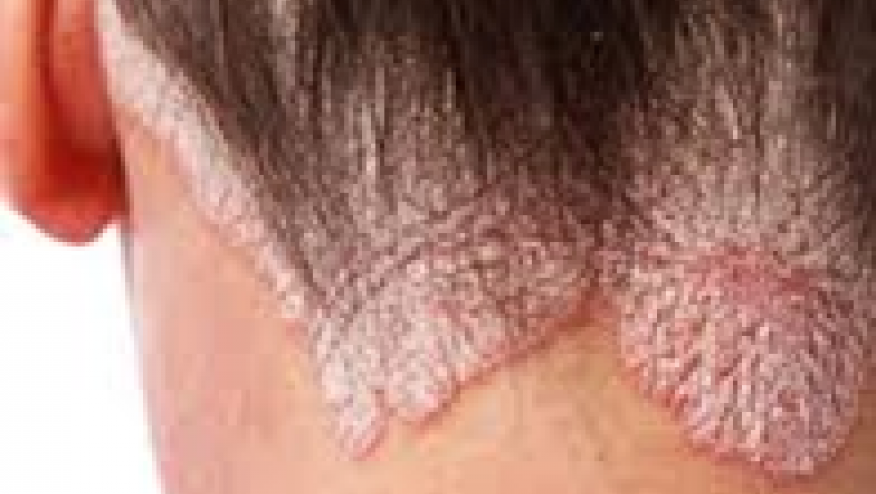
The IMMerge trial has demonstrated the superiority of interleukin (IL)‐23 over IL-17A inhibition adults with plaque psoriasis.
This multicenter, phase 3, open‐label study enrolled 327 adult patients with chronic, moderate‐to‐severe plaque psoriasis who were randomised risankizumab (RIS) 150mg or secukinumab (SEC) 300mg for 52 weeks. The primary efficacy end point was the Psoriasis Area Severity Index (PASI 90) at week 16 . (noninferiority comparison with margin of 12%) and week 52 (superiority comparison).
RIS was noninferior to secukinumab at week 16 with a PASI90 of 73∙8% vs. 65∙6%uperior to secukinumab.
By week 52 RIS was superior to SEC with a PASI90 of 86∙6% vs. 57∙1% (P<0∙001)], thussi meeting both primary end points.
No new safety concerns were identified.
The IL-23 inhibitor, risankizumab, is FDA approved for use in adults with moderate to severe plaque psoriasis.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.