US Trends in RA DMARD Use 2017-2021 Save
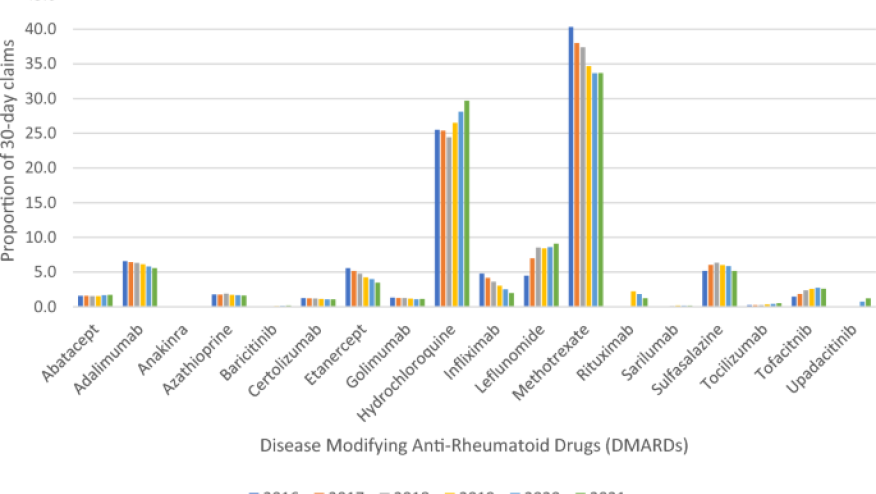
A trend analysis of disease-modifying anti-rheumatic drugs (DMARDs) shows that only half of rheumatoid arthritis (RA) patients are taking DMARDs, and that the COVID-19 pandemic substantially affected DMARD use. Despite numerous DMARD advances and evolving treatment guidelines since 1990, older therapies are still commonly used and the latest agents make up a small percentage of overall DMARD use.
This retrospective study utilized a commercial insurance claims dataset (Optum’s Clinformatics®Data Mart Database) to assess trends in the prevalence of DMARD use and new DMARD starts in the USA between 2016 to 2021 among RA adults. This analysis included conventional (csDMARDs), biologics [tumor necrosis factor (TNFi) and Non-TNFi), and Janus kinase inhibitors (JAKs)], and triple therapy [methotrexate (MTX), hydroxychloroquine (HCQ), sulfasalazine (SSZ)] use.
Among 670,679 commercially insured patients, the analysis included 1,381,758 patients with an RA and roughly 100,000 RA patients treated with a DMARD. The average age of the RA patients was 63.7 years (with 54% over the age of 65 years) and 76.7% were female and 70% were White. There was a 40% increase in unique patients per year from 2016 (88,826) to 2021 (123,278) and there was an increase in prescribed DMARDs from 46% (2019) to 51% (2020, 2021).
Trends included:
- csDMARDs remain the most prescribed (ranging from 77.2 to 79.2%)
- MTX was the most prescribed followed by HCQ and LEF
- JAKs were the least prescribed DMARD class, but their use doubled from 2016 (1.5%) to 2021 (4%). MTX
- During pandemic years: MTX use dropped (40% to 34% in 2021), and HCQ (< 25% to 30% in 2021) increased.
- significant decrease in infusion DMARDs in the pandemic era - infliximab (22% in 2019 to 15% in 2021) and rituximab (17% in 2019 to 12% in 2021)
- Triple DMARD therapy was very low (~ 1%)
- RA patients on biologics only (~ 17%) or biologics+MTX (~ 10%).
- 3 new bDMARDs were approved during the study period (upadacitinib 8/19; baricitinib 5/18, and sarilumab 5/17)
This cohort study is based on insurance claims and therefor includes patients not under the care of rheumatologists and thusly explains an older than expected RA population and a low number of RA patients on DMARDs. Clearly, the COVID-19 pandemic influenced DMARD use in the USA.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.