Ustekinumab Effective in Behcet's Disease Save
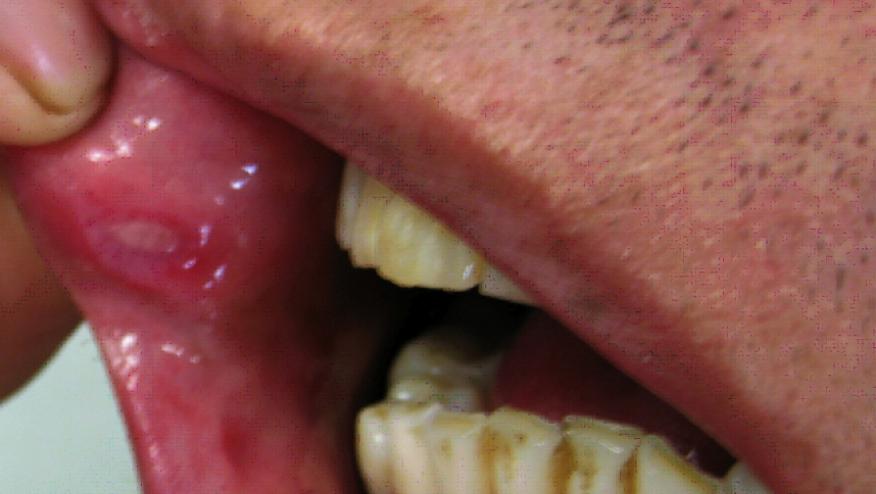
A small prospective study has shown that ustekinumab is safe and effective in Behçet's disease (BD) with recurrent oral ulcers (OU).
This was a multicenter open label study of 30 BD patients with active OU that was resistant to colchicine. Ustekinumab (90 mg subcutaneously) was given at at inclusion, week 4, and then every 12 weeks. The primary efficacy endpoint was the proportion of patients with complete response (no oral ulcers) at week 12.
The median number of OU per patient significantly decreased from 2 [2-3] to 0 [0-1], p<0.0001] by week 12.
Complete response was achieved in 60.0% and 88.9% at week 12 and 24, respectively.
The median Behçet's syndrome activity score (in which higher scores indicating a more active disease) was significantly lower at week 12 and 24 compared to baseline [70 [50-70] vs. 17.5 [10-42.5] and 10 [8-11], respectively, p<0.0001].
After a median of 12 months of follow-up 87% of BD patients were still receiving ustekinumab treatment.
These pilot data suggest the IL-12/23 inhibitor, ustekinumab to be effective in treating refractory BD oral ulceration and merits further study.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.