Legal (Not So) Merry-Go-Round of Alendronate Atypical Fractures Save
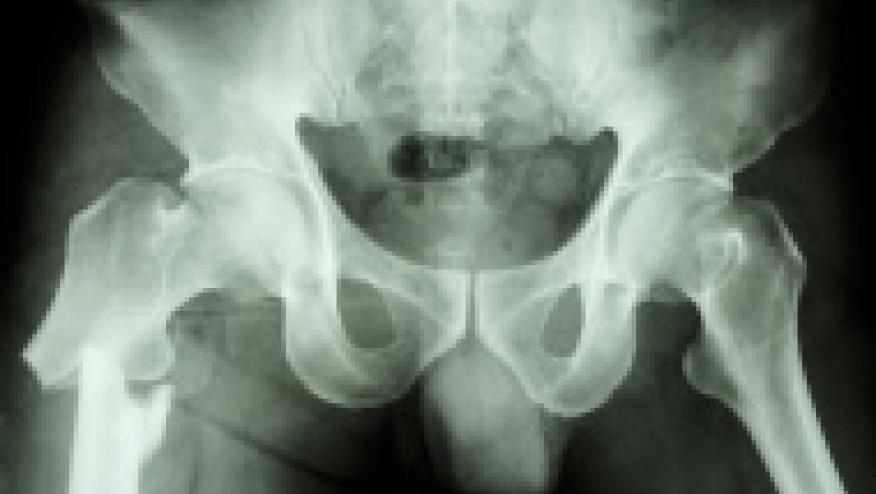
Dr. Gregory Curfman, MD (Executive editor of JAMA) writes on the chronological legal progress of a lawsuit stemming from rare instances of atypical femoral fractures associated with bisphosphonate (alendronate) use. While alendronate was FDA approved in 1995 for the prevention and treatment of osteoporosis, and legal action was initiated in 2011, there is still no legal resolution to this issue.
The following bullet points are excerpted from a well written "Viewpoint" in JAMA:
- subtrochanteric atypical fractures of the femur, unrelated to trauma are rare and thought to result from structural changes and potential instability in bone due to alendronate-induced reduction in the rate of bone turnover
- A lawsuit against Merck was initiated in 2011, a group of approximately 1000 plaintiffs with atypical femoral fractures while receiving alendronate
- Plaintiffs’ claim was that when the drug was approved (1995), until January 2011, the FDA-approved label did not include a warning of the risk of atypical femoral fractures, even though the manufacturer had been aware of the problem for years (a "failure-to-warn" liabity claim)
- The lawsuit against Merck (for 13 years) has been reviewed at all levels of the federal court system—district court, circuit court, and the Supreme Court; yet there is no legal resolution
- Merck argues that the plaintiff’s state (NJ) law claim is preempted by federal law; but to be preempted, Merck must have fully informed the FDA of the adverse and show that the FDA (having beien informed) must explicitly disapprove a change to the drug label to warn of atypical femoral fractures. (The injured plaintiffs argue that Merck never proposed a valid label change)
- 2008 Merck submitted a request to the FDA to update the alendronate label to include a warning about atypical femoral fractures. The FDA declined the label update as their reviewers were not persuaded by the evidence that atypical femoral fractures were caused by alendronate.
- Plaintiff point our that in FDA proposed updates, Merck referred to atypical femoral fractures as stress fractures and suggested that clinicians should consider other risk factors and causes
- FDA has made clear that it would consider a revised warning if Merck would use the proper terminology (atypical femoral fracture) and provide an accurate update to the alendronate label. Instead, Merck withdrew the proposed label change
- The US District Court for the District of New Jersey was the last to judge the case, and did not agree with the plaintiffs’ interpretation and ruled that the plantiffs' case was preempted
- Currently this is again on appeal to the United States Court of Appeals for the Third Circuit
- Merck (and the FDA?) are concerned that there can be risk of overwarning about the risk of rare adverse effects of drugs; but this works against full disclosure for doctors and patients
- The author and JAMA editor suggests..."the FDA may need to apply its statutory authority proactively to mandate label changes to appropriately warn patients and their physicians when warnings are indicated by clinical evidence."
- After 13 years in multiple courts at all levels of the federal judiciary, the plaintiffs in this case have not yet been provided a legal remedy for their injury.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.