Plasmacytoid Dendritic Cell Inhibitor in Cutaneous Lupus Save
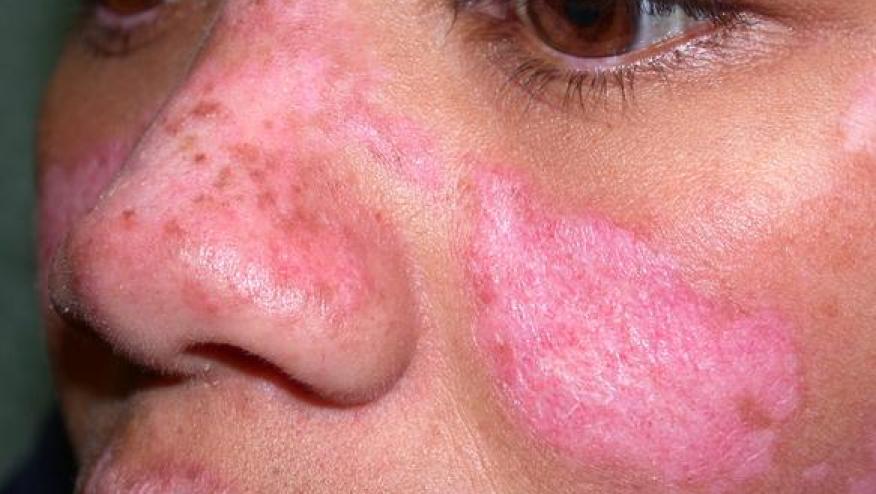
Litifilimab, a humanized monoclonal antibody against BDCA2, targets the BDCA2 receptor on plasmacytoid dendritic cells. When administered to patients with cutaneous lupus erythematosus (CLE) was shown reducing disease activity in CLE patients.
This phase 2 trial enrolled 132 adults with histologically confirmed CLE (with or without systemic manifestations) to receive placebo or 3 different doses of subcutaneous litifilimab (50, 150, or 450 mg) at weeks 0, 2, 4, 8, and 12. The Cutaneous Lupus Erythematosus Disease Area and Severity Index–Activity score (CLASI-A) score was used to assess outcomes; scores range from 0 to 70, with higher scores indicating more widespread or severe skin involvement.
The baseline mean CLASI-A scores and change (at wk 16) from baseline (compared to placebo) showed a significantly beneficial effect with the BDCA2 inhibitor.
| Dose Group | Baseline Value | Change in CLASI-A vs PBO |
| PBO | 15.2 | - |
| 50 mg | 18.4 | -24.3 |
| 150 mg | 16.5 | -33.4 |
| 450 mg | 16.5 | -28.0 |
Most of the secondary end points did not support the results of the primary analysis.
Litifilimab was associated with hypersensitivity and oral herpes infection (3 cases each) and one case of herpes zoster infection. One case of herpes zoster meningitis occurred 4 months after the participant received the last dose of litifilimab.
This early phase 2 trial suggests a clinical benefit for litifilimab in CLE, but larger trials are needed to better demonstrate efficacy. The number of herpes related adverse events is of concern in this short trial.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.