AGA Guideline: Prevention and Treatment of Hepatitis B Virus Reactivation in At-Risk Individuals Save
The American Gastroenterology Association has published its revised clinical practice guidelines for the prevention and treatment of hepatitis B virus (HBV) reactivation in at-risk patients, particularly those with immune-mediated disease, receiving immunomodulatory therapy and steroids. They note that antiviral prophylaxis is effective in lowering the risk of HBV reactivation, but in some, clinical monitoring without antiviral prophylaxis is sufficient.
An expert panel reviewed the clinical practice questions and scenarios, medical literature and use GRADE (Grading of Recommendations Assessment, Development and Evaluation) methodology to assess evidence and make recommendations. This new document is a revision of the first version guideline published in 2014.
Overall there were 4 recommendations.
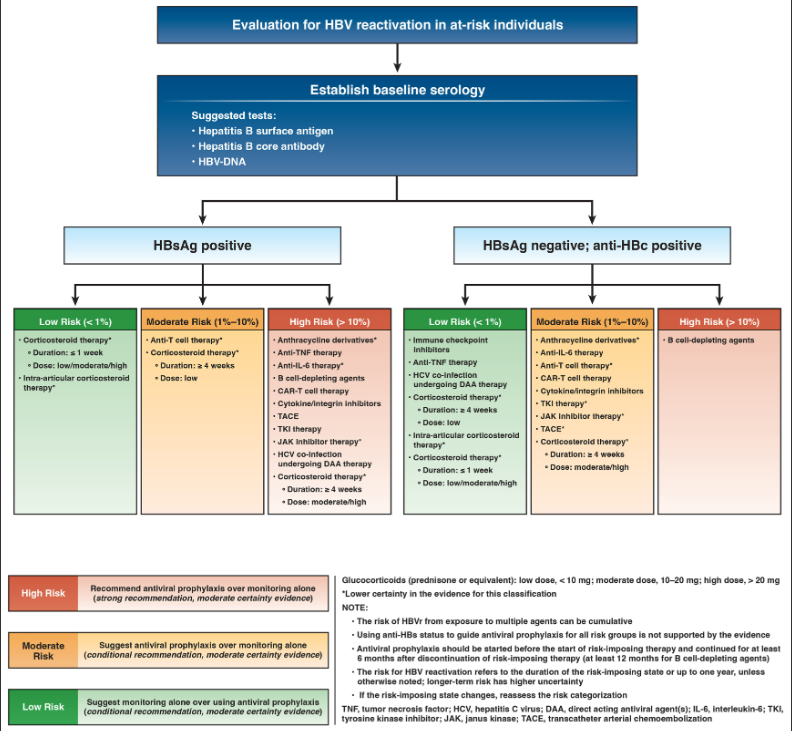
- The panel made a strong recommendation in favor of antiviral prophylaxis for individuals at high risk of HBVr.
- For individuals at moderate risk of HBVr, a conditional recommendation was made in favor of antiviral prophylaxis.
- For individuals at low risk of HBVr, a conditional recommendation favored monitoring alone without antiviral prophylaxis. Monitoring should be performed at 1- to 3-month intervals, and must include assessment of hepatitis B viral load in addition to assessment of alanine aminotransferase.
- For those at-risk of HBVr, a strong recommendation in favor of testing for HBV (CDC screening guidance: testing for HBV surface antigen, hepatitis B surface antibody, and total hepatitis B core antibody)
This document provides updated guidance for the management of HBVr in at-risk individuals (see full document for details).
The panel also made recommendations regarding specific anti-rheumatic agents:
- Corticosteroids: risk of HBVr with corticosteroid therapy is influenced by the dose and duration of systemic corticosteroid therapy. The highest risk of HBVr is seen with high-dose therapy when administered for ≥4 weeks. Short duration (< 1 week), low dose CS and intraarticular steroids are low risk for HBVr.
- TNF inhibitors: HBVr risk is based on 4 nonrandomized studies and 1555 patients.There is a low risk (<1%) of HBVr in HBsAg-negative/anti-HBc–positive anti-TNF therapy (2 per 1000). Incontrast, high risk of HBVr is seen among HBsAg-positive individuals on anti-TNF therapy (332 per 1000).
- Immune checkpoint inhibitors: HBVr risk in HBsAg-negative/anti-HBc–positive ICI patients was <0.1% (low risk). A “moderate risk” of HBVr is seen in HBsAg-positive/anti-HBc–positive on ICI therapy(70 per 1000).
- Ustekinumab/secukinumab: HBVr risk among HBsAg-negative/anti-HBc–positive patients on these agents was 13 per 1000 (“moderate risk”). In HBsAg-positive patients the risk of HBVr was 260 per 1000 (“high risk”). Based on limited studies and follow up.
- IL-6 Inhibitors: Limited data based on patients, steroid cotherapy and prior DMARD use suggests low risk of HBVr (<0.1%), based largely on HBsAg-negative/anti-HBc–positive patients on anti-IL6 therapy.
- JAK inhibitors: Based on limited data the risk in HBsAg-negative/anti-HBc–positive patients, as well as HBsAg-positive patients, was downgraded from moderate to low due to imprecision in the estimate of the baseline risk.
- Methotrexate, 6-mercaptopurine, and azathioprine: Literature does not provide an estimate of baseline HBVr in patients using using azathioprine, 6-MP use or methotrexate. AZA and 6MP does not have a significant impact on antibody responses. These medications have been in use for decades without HBVr cases directly attributable to these agents. The estimate the risk of HBVr with monotherapy to be <1%.
- Chimeric antigen receptor T cell therapy: from 7 studies all studies, all CAR-T patients who were HBsAg-positive were on antiviral prophylaxis (expected in this population - leukemia, lymphoma, and myeloma) and many included B cell–depleting agents such as rituximab, which classifies them as a high-risk group. Based on a pooled sample of 161 patients who were HBsAg-negative/anti-HBc–positive patients on CAR-T cell therapy, HBVr was seen 21 per 1000 (“moderate risk”). The panel classified HBsAg-positive patients undergoing CAR-T cell therapy to be at a “high risk” of HBVr.
Recommendations regarding antiviral prophylaxis is recommened for at least 6 months after cessation of exposure . However, in cases when the risk of HBVr is considered high, extension of antiviral therapy to 12 months is reasonable. In cases of exposure to B cell–depleting agents, antiviral prophylaxis should be extended to at least 12 months after end of exposure to B cell–depleting agents.
Join The Discussion
there appears to be a discrepancy in the recommendations. in the body of the report it indicates il-6 and hbsag neg, hb core + suggests low risk on hnvr; the algorithm above suggests moderate risk.
same with jak inhibitors.
Disclosures
The author has no conflicts of interest to disclose related to this subject










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.