PIANO Study - Safety of Biologics in Pregnancy Save

The results of the long awaited PIANO study have been reported in the journal Gastroenterology, showing that pregnant women with inflammatory bowel disease (IBD) may safely receive biologic or thiopurine therapy throughout the pregnancy without substantial added risk to the unborn or mother.
The PIANO study begain in 2007 as a cooperative study, formed by leading IBD devoted gastroenterologists, to study the effects of drug use during pregnancy in IBD patents; as many IBD patients will continue to need drug therapy to control disease activity during pregnancy. They enrolled 5 cohorts of pregnant IBD patients based on whether they were unexposed (n=379) or receiving thiopurines (azathioprine or 6-mercaptopurine, n=242), biologics (n=642) or a combination of thiopurines and biologics (n=227). Biologics included anti-TNF, anti-integrin, anti-IL 12/23 therapies. The primary outcomes included congenital malformations, spontaneous abortions, preterm birth, low birth weight (LBW) and infant infections.
From a total of 1490 completed pregnancies, there were 1431 live births. One-year infant outcomes were available in 1010. Overall, drug exposure did not increase the rate of congenital malformations, spontaneous abortions, preterm birth, LBW, and infections over the first year of life (compare to the unexposed).
Higher disease activity was associated with risk of spontaneous abortion (HR 3.41, 95% CI 1.51-7.69) and preterm birth with increased infant infection (OR 1.73, 95% CI 1.19-2.51). Use of a biologic was not associated with increased infection in the first year of life. Interestingly they noted that UC and CD mothers who flared during the 1st or 3rd trimester, were more likely to not be taking biologics or immunomodulators.
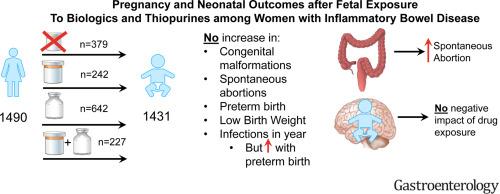
Key outcomes include 9% with congenital malformations, 3% with spontaneous abortion, 7% low birth weight and 10% preterm births. These did not differ between patients with Crohn's or ulcerative colitis.
Thus, biologic, thiopurine, or combination therapy exposure during pregnancy was not associated with increased adverse maternal or fetal outcomes at birth or within the first year of life.
Therapy with these agents can be continued throughout pregnancy in women with IBD to maintain disease control and reduce pregnancy related adverse events.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.