Updated EULAR Recommendations on the Treatment of Systemic Sclerosis Save
Medscape has published an overview to the 2024 updated recommendations for the treatment of systemic sclerosis (SSc) presented in Vienna at EULAR 2024 by Professor Francesco Del Galdo, MD, PhD on behalf of a 27 member task force.
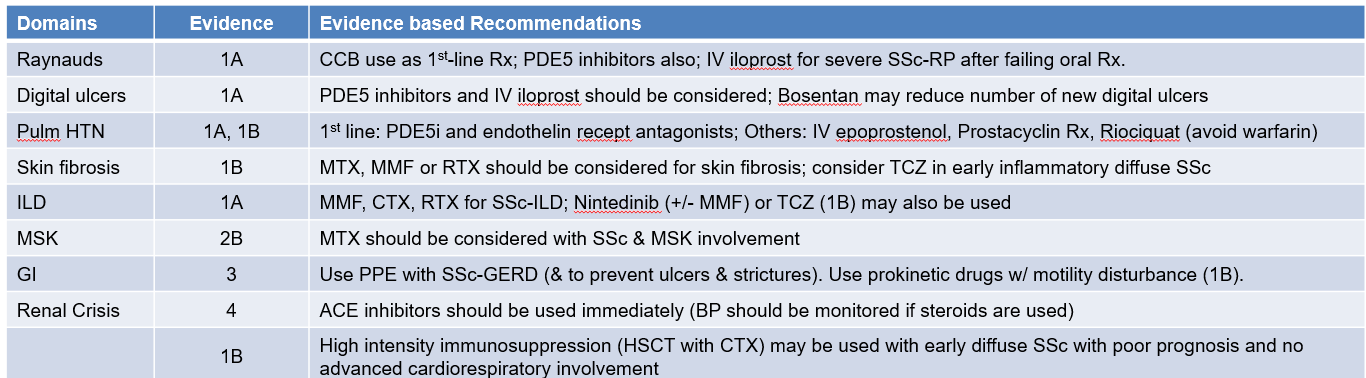
The new recommendations (n=20) supercede the 2017 (n=16) EULAR recommendations, providing specific evidence based suggestions for 8 specific domains of SSc (Raynauds, digital ulcers, Pulmonary hypertension, skin fibrosis, interstitial lung disease [ILD], musculoskeletal involvement [MSK], gastrointestinal [GI], and renal crisis).
"The most impactful new recommendation relates to the evidence for immunosuppressive agents and antifibrotics for the treatment of skin fibrosis and lung fibrosis," said Francesco Del Galdo, MD, PhD, professor of experimental medicine, consultant rheumatologist, and scleroderma and connective tissue diseases specialist at Leeds Teaching Hospitals NHS Trust, Leeds, England. Del Galdo presented the update at the EULAR 2024 Annual Meeting.
"But there are also new recommendations, including a redefined target population for hematopoietic stem cell transplantation following cyclophosphamide, the upfront combination treatment at the time of diagnosis of pulmonary arterial hypertension [PAH], and a negative recommendation for the use of anticoagulants for pulmonary arterial hypertension," noted Del Galdo, highlighting key updates in the 2024 recommendations.
Six new recommendations appear, covering drugs like mycophenolate mofetil, nintedanib, rituximab, and tocilizumab. None of these therapies were present in the 2017 recommendations.
Del Galdo highlighted the new immunosuppression continuum and associated treatments for skin and lung fibrosis. "For skin involvement, the task force recommended mycophenolate, methotrexate, and rituximab, with tocilizumab having a lower level of evidence and lower recommendation strength; similarly, in interstitial lung disease, we have rituximab, mycophenolate, cyclophosphamide, and nintedanib, and these all have the highest strength of evidence. Tocilizumab is assigned one strength of evidence below the other drugs."
He also cited the phosphodiesterase 5 inhibitor (PDE5i) drugs that are used across Raynaud's phenomenon, digital ulcers, and pulmonary arterial hypertension, which together form a vascular therapeutic continuum.
The complexity of systemic sclerosis and multiple manifestations was a major determinant of the recommendations, Del Galdo pointed out. "The task force realized that since this is such a complex disease, we cannot recommend one treatment unconditionally. For example, with mycophenolate mofetil, what works for most patients for the skin and lung manifestations might not for someone who experiences severe diarrhea, in which mycophenolate is contraindicated. So, the highest degree of recommendation that the task force felt comfortable with was 'should be considered.'"
Turning to new evidence around drug use, Del Galdo said that rituximab has the highest level of evidence across skin and lung manifestations, nintedanib is new in lung, and tocilizumab is new across both skin and lung.
To treat systemic sclerosis-pulmonary arterial hypertension (SSc-PAH), as long as there are no contraindications, the task force recommends using PDE5i and endothelin receptor antagonists (ERAs) at diagnosis. Data from phase 3 trials show a better outcome when the combination is established early.
The task force suggests avoiding the use of warfarin in PAH. "This is supported by a signal from two trials showing an increase in morbidity and mortality in these patients," noted Del Galdo.
He also pointed out that selexipag and riociguat were new and important second-line additions for the treatment of PAH, and —consistent with the ERA approach — the EULAR recommendation supports frequent follow-up to establish a treat-to-target approach to maximizing clinical outcomes in SSc-PAH and SSc-ILD. "Specifically, for the first time, we recommend monitoring the effect of any chosen intervention selected within 3-6 months of starting. The evidence suggests there is a group of patients who respond and some who respond less well and who might benefit from a second-line intervention."
For example, results of one trial support the approach of adding an antifibrotic agent to reduce progression in people with progressive lung fibrosis. "Similarly, for pulmonary hypertension, we recommend putting patients on dual treatment, and if this fails, place them on selexipag or switch the PDE5i to riociguat," Del Galdo said.
Recommendations by SSc domains are summarized in the table below.









If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.